Bioherb -
Chronic Hepatitis B Infection
Hepatitis B e antigen negative and e antibody positive
Recovery with Liv-Herbal Formula

( ) normal Range
Patient's liver enzymes are monitored based on blood work done at BC Biomedical Laboratories Ltd. and quantitative viral DNA count and PCR were done at the University of British Columbia, Virology and Reference Laboratory at St. Paul's Hospital, Vancouver B.C., Canada.
Fatty liver with border line Splenomegaly
Recovery with Liv-Herbal Formula
Patient diagnosed with chronic Hepatitis C (genotype 3) infection. Ultra Sound shows Fatty liver with border line Splenomegaly. Elevated liver enzymes and disturbance in liver function. Quantitative Hep-C RNA by PCR indicates 320,900 copies/ml. After two months of taking Liv-Herbal Formula a 2 log (98.84%) reduction in viral RNA copies/ml was achieved.

( ) figures indicate the normal values or range
Note: Viral genotyping, PCR, quantitative viral count were done at standard laboratories and Hospital.
(Liver Biopsy suggested possibility of Cirrhosis)
(Portal Hypertension and risk of Esophageal Varices)
Recovery with Liv-Herbal Formula in combination with Pegylated Interferon and Ribavirin therapy
Patient with history of chronic Hepatitis C (genotype 1a) infection. Liver Biopsy suggested the possibility of Cirrhosis. Evidence of Portal vein Hypertension and risk of Esophageal Varices. After a month of Interferon treatment the PCR was Positive for Hep-C viral RNA presence and after 3 months of Interferon therapy patient barely achieved a 2-log drop in viral load. At this time patient started Liv-Herbal Formula. It is reported that Hepatitis C virus genotype 1a does not respond to therapy that well but in this case the 6th month blood test indicated as Negative (No viral RNA was detected in the blood). It means that 3 month of taking Liv-Herbal Formula has helped the patient to achieve the results that doctors did not think is possible. This finding suggests that Liv-Herbal Formula definitely contributed to patient's recovery and is safe if taken along Interferon and anti-Viral drug therapy.
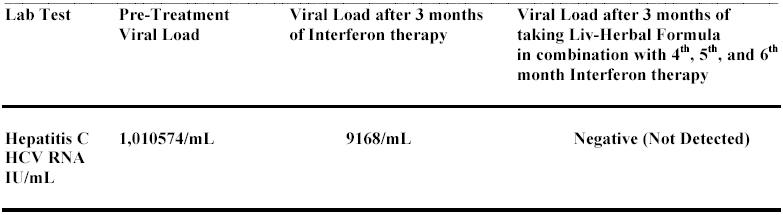
Blood work, Biopsy, Serology and PCR were done at standard laboratories and Hospitals in Canada.
Mildly enlarged liver and portal vein hypertension
Swollen kidneys with malfunction
Recovery with Liv-Herbal Formula
Patient in mid thirties with Chronic Hepatitis C, mildly enlarged liver, advanced Cirrhosis and Portal Vein Hypertension, swollen kidneys and kidney malfunction. Hospitalized for 3 weeks in the ICU. Liver transplant was suggested.

( ) figures indicate the normal values or range
Blood work, PCR and quantitative viral count were done at standard laboratories and Hospitals.Note: The above data indicates that the patient's liver is recovering and its function has also improved. Patient's Kidneys are normal in size and function after treatment and does not need Liver Transplant. A 98.8 % reduction in Hepatitis C Viral RNA copies/ml of blood was observed after taking Liv-Herbal Formula. No other therapy was given.
Relapse after six months course of Interferon therapy
Patient in third trimester of pregnancy
Recovery with Liv-Herbal Formula
Patient with history of chronic Hepatitis C infection in the third trimester of pregnancy. Six months course of Interferon therapy did not clear the virus in this case. Prior to taking Liv-Herbal Formula the Hep-C RNA load was 85000 copies per ml and after 45 days of taking Liv-Formula viral count dropped to 11000 copies per ml (a reduction of 87%).
 Note: Blood work, PCR and quantitative viral count were done at standard laboratories.
Note: Blood work, PCR and quantitative viral count were done at standard laboratories.Data presented here is selected from the research files of Bio-Herb Remedies Inc. Canada
Note: Canada has a national health care plan that covers patients' medical cost. Immediate or frequent medical tests are requested only when physicians consider it is essential, otherwise, they are done every three to six months, or yearly. When the data presented here indicates improvement after 6 or 9 months or years of taking Liv-Herbal Formula, it does not necessarily mean that one has to take it for that long to see an improvement - the medical tests in these cases were done after a longer period of time.